No Person or artificial algorithm or even all money and all the smartest people in the world together have the capacity or the potential to create a vaccine in 100 days that is safe and effective!
Nobody and nothing can do this!
Because the timeframe is the Clou of safe and effective that allows the professional and carefully acting scientist to study and proof the material of his outcomes.
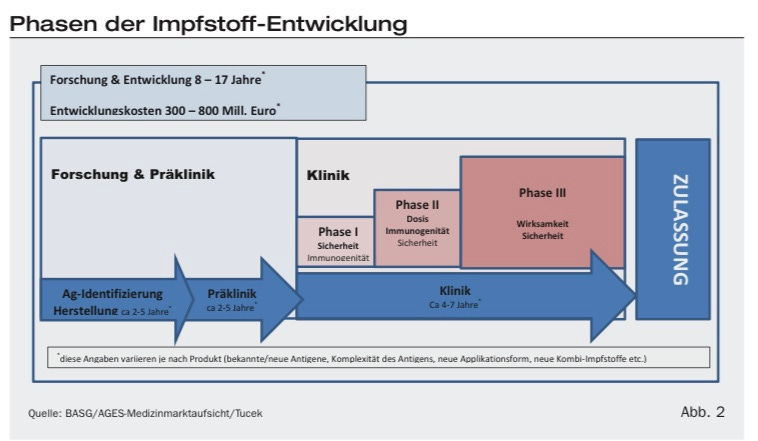
To Take Time means you take care and that costs a lot of money, clearly!
So you can’t change the direction and put the same money in a shorter time periode to have the same Result of Quality!
Thats is physically Impossible and in the real world of good manufacturing practice Undisputed!
Quantity and Quality are firmly connected to each other in an inesitable relationship. Like the Moon to the Earth or Quantum mechanics. That means if you change a composition on the one side you always change the direction at the other side!
Why we have reached the point where we have forgotten the simplest things such as logical thinking and lost touch with reality?
Medical inventor and author David Martin proves that the Pfizer and Moderna mRNA vaccines are not vaccines by medical definition and how Big Pharma is using national and state emergency authorizations to force these untested gene-chemo-therapies onto the population.
https://odysee.com/@ARGONAUT:d/davidmartin:8
Dr David E Martin is the Founder and Chairman of M-CAM International, RASA Energy, and is a Batten Fellow of the University of Virginia. His experience includes founding the first medical device clinical trials center at UVA while on the medical school faculty in radiology and orthopedic surgery. His work with global investigation of biological and chemical weapons was done through his company Mosaic Technologies in which he led numerous defense to civilian technology transfer programs with the world’s largest companies and countries. His work includes publications and academic appointments in medicine, law, and economics. His firm M-CAM was responsible for leading investigation and supporting prosecution of some of the largest financial frauds in U.S. history as a contractor for the U.S. Treasury.
David E. Martin Speaker, Author, Fully Human
‘Butterfly’ of the Week: Hidden in Plain Sight…
References
It is unlawful under the FTC Act, 15 U.S.C. § 41 et seq., to advertise that a product or service can prevent, treat, or cure human disease unless you possess competent and reliable scientific evidence, including, when appropriate, well-controlled human clinical studies, substantiating that the claims are true at the time they are made.
Definition of Vaccine https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm
Immunity: Protection from an infectious disease. If you are immune to a disease, you can be exposed to it without becoming infected.
Vaccine: A product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that
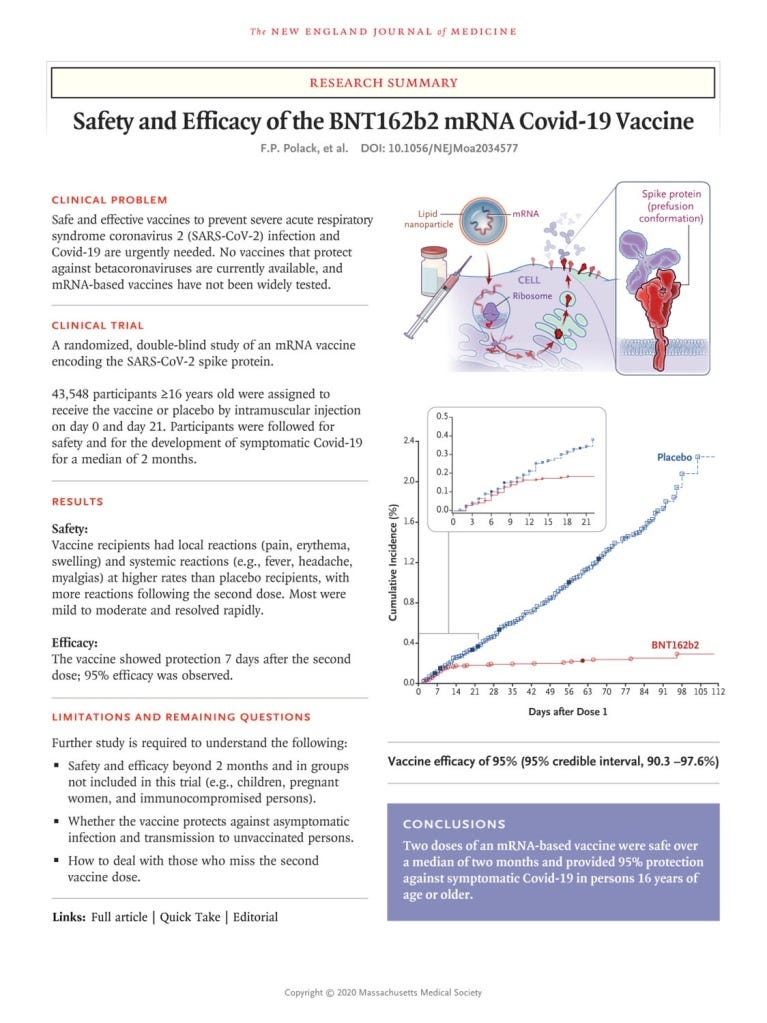
disease. Vaccines are usually administered through needle injections, but can also be administered by mouth or sprayed into the nose.“The primary endpoint is the prevention of symptomatic COVID-19 disease. Key secondary endpoints include prevention of severe COVID- 19 disease and prevention of infection by SARS-CoV-2.” https://investors.modernatx.com/news-releases/news-release-details/modernas- covid-19-vaccine-candidate-meets-its-primary- efficacy#:~:text=About%20the%20Phase%203%20COVE%20Study&text=The%20primary%20endpoint%20is%20the,by%20SARS%2DCoV%2D2.
“As of this writing, no correlate of protection for SARS-CoV-2 has been established.” https://www.nejm.org/doi/full/10.1056/NEJMoa2028436
“No existing vaccines have been shown to be effective against infection with any betacoronavirus, the family that includes SARS-CoV-2, which causes Covid-19.” Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615.
Dave wrote his first classified briefing about bio-weapons corruption in 2001, and has been presenting lectures at bio-weapons conferences since then. He has been tracking Dr Anthony Fauci’s spending and notes that Fauci has authorised $191 billion in funds for the bio-weaponisation of viruses against humanity.
In April 2021 he published the Fauci Dossier public document, 205 pages, 22 years of research for humanity to use and prosecute, and in November 2021 his presentation SLIDES contained the COVID profiteers.
Please watch David’s excellent speech at the European Parliament in Strasbourg on 13 September 2023 here
https://twitter.com/i/status/1702717102787027098
If the WHO Pandemic Agreement passes in May, (in a nut shell) the WHO will gain the power to “suspend all civil liberties” in the event that it arbitrarily decides to declare a public health emergency of international concern (PHEIC)
“[Covid was used to] terrorise the world, convince them that we need some giant protector state that actually has some sort of supranational ability, and then suspend civil liberties as long as they need to be suspended… at the whim of funding agencies who have no criminal accountability.“
“These things are set up to be terror campaigns, to modify the public’s willingness to give up their liberties.”Dave Martin
There doesn’t have to be any evidence for the WHO to declare and emergency, there is no standard for what constitutes the emergency, and they have complete liability protection. “They wrote absolute immunity from all prosecution into their charter“
In respect of words spoken or written or acts done by them in the performance of their official functions, immunity of legal process of every kind, such immunity to continue notwithstanding that the persons concerned are no longer serving on committees of, or employed on missions for, the Organization;WHO Constitution: CONVENTION ON PRIVILEGES AND IMMUNITIES, Annex VII clause 2(i)(b), Page 39 – PDF
Lets follow some facts…
Science and human behavior
Burrhus Frederic Skinner, Simon and Schuster, 1965
The psychology classic—a detailed study of scientific theories of human nature and the possible ways in which human behavior can be predicted and controlled—from one of the most influential behaviorists of the twentieth century and the author of Walden Two.“This is an important book, exceptionally well written, and logically consistent with the basic premise of the unitary nature of science. Many students of society and culture would take violent issue with most of the things that Skinner has to say, but even those who disagree most will find this a stimulating book.”—Samuel M. Strong, The American Journal of Sociology “This is a remarkable book—remarkable in that it presents a strong, consistent, and all but exhaustive case for a natural science of human behavior… It ought to be… valuable for those whose preferences lie with, as well as those whose preferences stand against, a behavioristic approach to human activity.”—Harry Prosch, Ethics
Vaccinegate:
MRC-5 contained in Priorix Tetra – Complete genome sequencing
Remdesivir the first Emergency Use authorized Medical Product by the FDA or CDC not only haven’t any benefit, but it also has a horrific safety profile.
Conclusions: Deterioration of liver and kidney function are frequently observed ADEs with remdesivir!
Of course, the infamous Ebola trial that showed the true deadly profile of this drug:
“However, six months into the Ebola study, the trial’s Safety Review Board suddenly pulled both remdesivir and ZMapp from the trial. 12 Remdesivir, it turned out, was hideously dangerous. Within 28 days, subjects taking Remdesivir had lethal side effects including multiple organ failure, acute kidney failure, septic shock, and hypotension, and 54 percent of the remdesivir group died—the highest mortality rate among the four experimental drugs. 13”
Combine renal failure with intubation and that is what padded the “excess deaths” of the Covid-19 “pandemic.”
Therefore, when the MSM comes out with claims that Remdesivir has no benefit, what they are really doing is engaging in a coverup. BigPharma, FDA, CDC, WHO, NIH, Pentagon, et al. all knew the true “safety” profile of this deadly drug.
No budget in the world has the competence to create safe and effective vaccines from 10-15 Years of safty studys and correction to 100 days to handout! Thats not only 100% unscientific but it’s also 100% unprofessionally and Negligent .
“IBM was the solutions company and their mandate was to bring in any solution the customer wanted, including the final solution,” he says. “IBM continues to cling to the hope that the world will forget that it co-planned and co-organized all six stages of the holocaust.”
The prisoner, classified as a misfit or “asocial,” according to the Germans, was located at the Dachau concentration camp. The decoding key shows the prisoner could have also been classified as a homosexual, Jew, or Gypsy. There were also other options describing the “method of departure.”
IBM now has an executive dedicated to corporate citizenship and is recognized by many as one the world’s most ethical and progressive companies. Its Corporate Service Corps program puts exceptional employees into developing countries to work on local projects to improve water quality and disaster preparedness. IBM has been ranked first by Business Ethics magazine on its annual top 100 Best Corporate Citizens list, and in 2011 Corporate Responsibility Magazine’s list ranked it as the third best corporate citizen.
I swear they did it again they have the motiv the used the tactics abd they have the history to be complicit
Cleveland Clinic and IBM Unveil First Quantum Computer Dedicated to Healthcare Research
IBM Quantum System One deployed at Cleveland Clinic as part of landmark 10-year partnership
So they and machine can decide who may live and who must die all by the algorithms!That’s horrific!
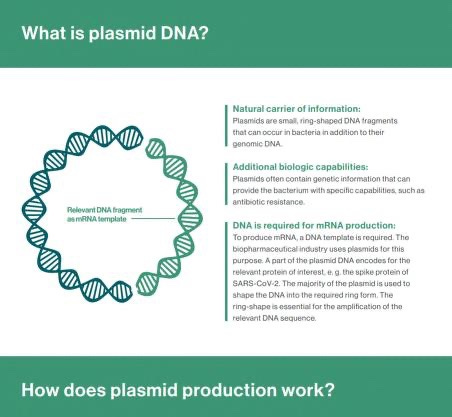
Some documents about plasmids and the dna contaminations of the so called COVID-19 vaccines
THE ROLE OF PLASMID CONSTRUCTS CONTAINING THE SV40 DNA NUCLEAR-TARGETING SEQUENCE IN CATIONIC LIPID- MEDIATED DNA DELIVERY
TEKKATTE KRISHNAMURTHY PRASAD and NALAM MADHUSUDHANA RAO*
Centre for Cellular and Molecular Biology, Uppal Road, Hyderabad (AP), India 500007
Abstract: One of the steps that limit transfection efficiency in non-viral gene delivery is inefficient nuclear import of plasmid DNA, once it has been delivered into the cytoplasm. Recently, via microinjection into the cytoplasm and in situ hybridizations into a few cell types, it was shown that a region of Simian virus 40(SV40), specifically a c. 372-bp fragment of SV40 genomic DNA encompassing the SV40 promoter-enhancer-origin of replication (SV40 DTS), could enable the nuclear import of a plasmid carrying these sequences (Dean D.A. Exp. Cell Res. 230 (1997) 293). In this report, we address the issue of the suitability of the SV40 DTS for cationic lipid-mediated gene delivery, and its capacity to improve the efficiency of the transfection process. For this study, we used transient reporter gene expression assays on various cell types. The gene expression from the plasmid constructs carrying the SV40 DTS varied with cell type and plasmid construct used. Such cell-type and plasmid-construct dependency on gene expression from plasmids containing the SV40 DTS suggests that the gene expression from plasmids is not entirely dependent on its ability to enhance the nuclear import of said plasmids.
Corresponding author, tel: +91-40-7192552, fax: +91-40-7160591/ 7160311 e-mail: madhu@ccmb.res.in
Abbreviations used: β-gal – β-galactosidase; CLDC – cationic lipid DNA complexes; Chol – cholesterol; CMV – Cytomegalo virus; DHDEAB – N,Ndi-( n-hexadecyl)-N,N- di(hydroxyethyl)ammonium bromide; DTS – DNA nuclear targeting sequence; DMEM – Dulbecco’s modified eagles medium; HEPES-HBSS – HEPES buffered Hank’s balanced salt solution; EBNA1 – EBV nuclear antigen 1; EBV – Epstein Barr virus; luc or lux – Luciferase; NLS – nuclear localization sequence; NPC – nuclear pore complexes; PBS – phosphate buffered saline; SV40 DTS – SV40 promoter-enhancer-origin; SV40 – Simian virus 40; TNF-α − tumor necrosis factor-α; TPA – 12-O-tetradecanoyl-phorbol -13- acetate.
https://www.respiratorygenetherapy.org.uk/
Emerging significance of plasmid DNA nuclear import in gene therapy.
Munkonge FM, Dean DA, Hillery E, Griesenbach U, Alton EW
Advanced Drug Delivery Reviews
Adv Drug Deliv Rev. 2003 Jun 16;55(6):749-60.
Print this page
The signal-mediated import of plasmid DNA (pDNA) into nondividing mammalian cell nuclei is one of the key biological obstacles to nonviral therapeutic pDNA delivery. Overcoming this barrier to pDNA transfer is thus an important fundamental objective in gene therapy. Here, we outline the rationale behind current and future strategies for signal-mediated pDNA nuclear import. Results obtained from studies of the nuclear delivery of pDNA coupled to experimentally defined nuclear localisation signal (NLS) peptides, in conjunction with detergent-permeabilised reconstitution cell assays, direct intracellular microinjection, cell-based transfection, and a limited number of in vivo experiments are discussed.
Go Back to Publications View on Pubmed (12788538) View on Google Scholar
Other Publications by these authors
Identification and functional characterization of cytoplasmic determinants of plasmid DNA nuclear import.
Munkonge FM et al. The Journal Of Biological Chemistry (2009) Assessment of CFTR function after gene transfer in vitro and in vivo.
Griesenbach U et al. Methods In Molecular Biology (Clifton, N.J.) (2008) Anti-inflammatory gene therapy directed at the airway epithelium.
Griesenbach U et al. Gene Therapy (2000)Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators.
Munkonge FM et al. Journal of Cystic Fibrosis (2004)
MATERIALS AND METHODS
pCMVβ-gal SPORT and Lipofectamine2000 were purchased from Invitrogen, USA. pGL3-control, pGL3 basic and pBlueScript were obtained from Stratagene. pECFP-C1 was purchased from Clontech. Plasmid pCH110 and T4 DNA ligase was obtained from Amersham Pharmacia. T4 DNA polymerase was obtained from New England Biolabs. α 32P-dATP was purchased from Bhaba Atomic Research Centre, Mumbai, India. Qiagen DNA purification columns were purchased from Qiagen. NBD-PE was purchased from Molecular Probes (USA). All the primers used in this study were synthesized in-house using the Applied Biosystems 394 DNA/RNA synthesizer. All the other chemicals were obtained from Sigma Co, USA
Cell culture and transient transfections
All the cell lines used were maintained in DMEM, containing 10% fetal calf serum and antibiotics (Penicillin, Streptomycin and Kanamycin) in an incubator at 37°C and 5% CO2. Transient transfections were done as described before, using DHDEAB:Chol (1mM:1mM), whose synthesis, liposome preparation and characterization have been described [14], or Lipofectamine2000 according to the manufacturer’s protocol. Transfections were done by complexing plasmid DNA and lipid at 1:1 lipid:DNA charge ratios. A β-galactosidase assay was done, as described before, after 24 h of transfection [14]. β-galactosidase activity was calculated from a standard graph constructed for a commercial β- galactosidase enzyme. β-galactosidase activity was normalized against a mlligram of cell protein. The transfection of aphidicolin-treated cells was done as described above, except that the cells were treated with a 5 μg/ml final concentration of aphidicolin in 10% serum-containing medium 24 h before transfection and 21 h after the complexes were removed from the cells. Each assay was performed in triplicate. The reported data is representative of three completely independent experiments.
Nick translation of plasmid DNA and the kinetics of cell association and plasmid uptake
pCMV β-gal, pSV40enCMV β-gal and pSV40DTSCMV β-gal were labeled with α-P32-dATP by nick translation. 2.5 μg of each plasmid was used for nick translation. Nick translation was carried out according to the standard protocol [16]. For the plasmid uptake kinetics experiment, labeled plasmids were mixed with their respective cold plasmids such that each well of a 96-well plate should have about 10,000 counts and 0.3 μg of plasmid. Transient transfections were done as before. 1 h, 2 h, 3 h and 4 h after the transfection medium was removed, the cells were washed thrice with PBS and lysed in 50 μl of lysis buffer for 10 min. at room temperature. The percentage cell associated counts were then plotted as a function of time.
Quantitating internalized CLDC by Fluorescence Activated Cell Sorting (FACS)
To accurately quantitate the amount of plasmid DNA internalized by cells as CLDC, we prepared CLDC using DHDEAB:Chol (1:1 mol/mol) liposomes containing 5 mol% NBD-PE as a fluorescent marker. COS-1 cells were grown in 6-well plates to about 70%-80% confluency. Complexes were prepared with three different plasmids – pCMV β-gal, pSV40enCMV β-gal and pSV40DTS CMV β-gal at a 1:1 lipid:DNA charge ratio. Complexes containing about 1 μg of plasmid DNA were added to each well and incubated for about 3 h. After the incubation, cells were washed once with HEPES-HBSS. Cells were trypsinized and washed with HEPES-HBSS. The external fluorescence contributed by cell surface associated CLDC was quenched by adding 0.4% trypan blue containing HEPES-HBSS. The addition of trypan blue has been shown to efficiently quench the external fluorescence of CLDC [17-19]. Cells were then washed twice with HEPES-HBSS without trypan blue, and finally resuspended in the same buffer. Samples were run on a FACS Calibur, BD Biosciences, and 10,000 events were collected per sample. Cells without the addition of CLDC were run as a control. The data is expressed as the percentage of cells that were positive for CLDC uptake.
FACS analysis of aphidicolin-treated cells
CHO cells were grown in 35 mm dishes till they reached a confluency of about 60%. The cells were then washed with PBS and 2 ml of DMEM containing 10% serum was added, which either contained aphidicolin (5 μg/ml) or contained only DMSO of an appropriate concentration (control). The cells were incubated for 24 h at 37°C and 5% CO2. After 24 h, the medium was removed and the cells were washed twice with PBS. The cells were collected by trypsinization and washed with PBS to remove trypsin, and then were resuspended in cold 70% ethanol and stored at 4°C until they were processed for FACS analysis. The cells were washed twice with PBS and finally resuspended in about 0.8 ml of PBS. To this, about 0.1 ml of RNAse (1 mg/ml) and 0.1 ml of propidium iodide (400 μg/ml) were added, and tube was incubated at 37°C for 30 min. Samples were run on a FACS Calibur, BD Biosciences, and 10,000 events were collected per sample in a propidium iodide channel.
RESULTS AND DISCUSSION
For convenience, the details of all the plasmid DNA constructs used are shown in Tab. 1. Initially, we used three plasmid vectors – pCMV β-gal (pCMV β-gal- SPORT), pSV40enCMV β-gal and pSV40DTSCMV β-gal. pCMV β-gal is a control plasmid without SV40 DTS. pSV40enCMV β-gal includes only a SV40 72-bp repeat enhancer, hereafter referred to as partial SV40 DTS, and pSV40DTSCMV β-gal includes the SV40 promoter-enhancer-origin of replication, hereafter referred to as full-length SV40 DTS. We performed transient transfections in dividing COS-1, HeLa, CHO and NIH-3T3 cell types using a DHDEAB:Chol (1:1) cationic liposome formulation.
We observed a 2.5-fold increase in gene expression in the COS-1 cells given pSV40DTSCMV β-gal over its control plasmid, pCMVβ-gal (Fig. 1A). pSV40enCMV β-gal, which contains the 72-bp repeat enhancer sequence, did not give rise to any increase in expression compared to pCMV β-gal. There was no increase in gene expression with either pSV40enCMV β-gal or pSV40DTSCMV β-gal over the control plasmid pCMV β-gal, in CHO, HeLa and NIH-3T3 cells (Figs. 1B, C and D). In case of COS-1 and NIH-3T3 cells, pSV40enCMV showed less reporter gene expression compared to the control plasmid, pCMV β-gal. Transfections were repeated with Lipofectamine2000, a commercial transfecting agent, on COS-1 and NIH-3T3 cells with the three plasmids. There were no distinguishable differences in the data on transfection efficiencies for the three plasmids between Lipofectamine2000 and DHDEAB:Chol, indicating that the behavior of the plasmid is not dependent on the transfecting lipid (data not shown).
Fig. 1. Transient transfections with plasmid constructs. This panel shows plots of normalized milliUnits of β-galactosidase ativity from pCMV β-gal (1), pSV40enCMVβ- gal (2) and pSV40DTSCMVβ-gal (3) plasmid constructs in COS-1 (A), CHO (B), HeLa (C) and NIH-3T3 cells (D).
In order to see if the differential expression seen for pCMV β-gal, pSV40enCMV β-gal and pSV40DTSCMV β-gal in COS-1 cells is due to differential cell association and uptake of these plasmid constructs, we quantitated the kinetics of plasmid cell association and uptake as described above in the Materials and Methods section. Fig. 2A shows that all three plasmids were taken up to the same extent by COS-1 cells after transient transfection. We also carried out uptake experiments by quantitating only internalized complexes in COS-1 cells, as described in the Materials and Methods section. Fig. 2B shows that the uptake efficiency for the different plasmids is the same. We observed about 49.67%, 53.95% and 48.79% uptake efficiency for the pCMV β-gal, pSV40enCMV β-gal and pSV40DTS CMV β- gal plasmids, respectively. These experimental results indicate that the differences in the level of gene expression between these plasmid constructs is not due to differential internalization of these plasmids by the cells.
Aaron Siri, the lead counsel for ICAN, delivered compelling testimony at the Arizona Novel COVID South Western Intergovernmental Committee (NCSWIC), an important platform dedicated to addressing the challenges and lessons learned from the COVID-19 pandemic, and charting a path forward. From https://rumble.com/user/HealthrightsMA
Covid-19 Vaccine Expert Panel Briefing to the Massachusetts Legislature and Public Health Officials https://rumble.com/v48zr6o-covid-19-vaccine-expert-panel-briefing-to-the-massachusetts-legislature-and.html
https://julimination.wordpress.com/2024/05/14/this-is-not-a-vaccine-by-definition/
Respect Me&You & PlanetBlue 🕊